Kim jesteśmy?
SciencePharma (SPh) jest dynamicznie rozwijającą się polską firmą oferującą specjalistyczne usługi doradcze i importowe dla sektora farmaceutycznego. Na rynku funkcjonujemy od 2004 roku. Jesteśmy liderem wśród firm konsultingowych działających w Polsce i za granicą. Od kilkunastu lat realizujemy z powodzeniem projekty o zasięgu krajowym i międzynarodowym (EU, non EU). Na swoim koncie mamy m.in. przygotowanie kompletnej dokumentacji rejestracyjnej dla ponad 500 produktów leczniczych oraz wiele innych sukcesów i osiągnięć.
Naszą mocną stroną jest znajomość wymagań prawnych, wytycznych, procedur oraz oczekiwań agencji rejestracyjnych co do zakresu i jakości dokumentacji rejestracyjnej, a także bogate doświadczenie zdobyte dzięki realizacji każdego rodzaju projektów rejestracyjnych i rozwojowych na rzecz wielu firm farmaceutycznych.
Jesteśmy do dyspozycji zawsze gdy chcą Państwo skorzystać ze wsparcia i powierzyć swoje sprawy w ręce doświadczonych profesjonalistów.
Poza zagadnieniami związanymi z obsługą rejestracyjną, od kilku lat sami z powodzeniem całościowo realizujemy projekty rozwojowe leków.
Oferujemy wsparcie od etapu koncepcji aż do wdrożenia, uwzględniając etap rozwoju fizykochemicznego, badań in vitro poprzez badania niekliniczne i kliniczne, aż po rejestrację i zarządzanie produktem na rynku (utrzymanie pozwolenia, PharmacoVigilance).
Zaprojektowane przez nas badania kliniczne niejednokrotnie zapewniły powodzenie procedur rejestracyjnych, a nieszablonowe podejście i wytrwałość w dążeniu do celu zaowocowały wieloma zmianami kategorii dostępności z Rx na OTC.
Dzięki niebanalnym rozwiązaniom jesteśmy w stanie dostrzec różne możliwości, proponować alternatywne scenariusze działań, a przede wszystkim jesteśmy gotowi na bieżące reagowanie w niestandardowych sytuacjach.
Specjalizujemy się także w imporcie produktów leczniczych przeznaczonych na rynek oraz do badań klinicznych.
Nasi Eksperci mają szeroką wiedzę i doświadczenie w zakresie wymagań GMP/GDP. Setki godzin spędzone na prowadzeniu audytów miejsc wytarzania oraz masy dokumentów przeglądane podczas procesu certyfikacji produktów leczniczych procentują poprzez kształtowanie nas jako lidera w zakresie świadczenia usług importu produktów leczniczych i certyfikacji serii przez Osobę Wykwalifikowaną.
Jesteśmy posiadaczem zezwolenia na wytwarzanie i import przyznanego przez Głównego Inspektora Farmaceutycznego.
Nasz entuzjastycznie nastawiony, interdyscyplinarny zespół chętnie zaangażuje się w nawet najbardziej skomplikowaną sprawę, dbając o wysoką jakość i zadowolenie naszego Klienta.
Wśród naszych ekspertów są osoby o różnym wykształceniu i dorobku naukowym (farmaceuci, lekarze, chemicy, biolodzy, prawnicy) oraz bogatym doświadczeniu zdobytym m.in. w przemyśle farmaceutycznym, medycznym, w laboratoriach analitycznych, w Urzędzie Rejestracji Produktów Leczniczych i Wyrobów Biobójczych czy w Ministerstwie Zdrowia.


Nasze usługi
Poznaj bliżej profesjonalne usługi doradcze oferowane przez SciencePharma. Od ponad 18 lat wspieramy polskich i zagranicznych Klientów w zakresie rejestracji produktów leczniczych, nadzoru nad bezpieczeństwem farmakoterapii, audytów GxP, badań klinicznych i nieklinicznych oraz realizacji projektów badawczo-rozwojowych.
Specjalizujemy się również w imporcie produktów leczniczych mając w zespole ekspertów od wymagań GMP oraz osoby wykwalifikowane (QP). Oferujemy pełen wachlarz usług z zakresu jakości produktów leczniczych, w tym pomoc w transferach technologii, walidacji procesów wytwarzania czy też wdrażania systemu GMP. Służymy wsparciem w całym cyklu życia produktu, od badań rozwojowych po komercjalizację. Poniżej szczegółowe informacje dotyczące naszych usług.
To co nas wyróżnia na tle konkurencji to szeroki wachlarz obszarów działań. Oferujemy wsparcie nie tylko w kwestii produktów leczniczych (ludzkich i weterynaryjnych) – posiadamy doświadczenie również w zakresie wyrobów medycznych, produktów ATMP, konopi medycznych czy też kosmetyków i suplementów diety.
Nasze osiągnięcia
0
Projektów rozwojowych dla produktów leczniczych
0
Dossier rejestracyjnych (moduły 1-5) dla produktów leczniczych
0
Udanych rejestracji w procedurze krajowej oraz w procedurach europejskich MRP i DCP
0
Produktów leczniczych w naszym systemie Pharmacovigilance
0
Audytów GMP (EU i kraje trzecie)
0
Badań klinicznych i obserwacyjnych dla produktów leczniczych i wyrobów medycznych
Dlaczego my?
Liderzy w branży z ponad 20 letnim doświadczeniem
Światowy serwis
Profesjonaliści z multidyscyplinarnym doświadczeniem
Znajomość prawa farmaceutycznego
Dlaczego my?
Ponieważ projekty Klienta traktujemy jak nasze własne – z największym zaangażowaniem, nie boimy się wyzwań, a kultura naszej pracy nakierowana jest na Klienta. Doskonała znajomość prawa farmaceutycznego, doświadczenie we współpracy z krajowymi i międzynarodowymi agencjami rejestracyjnymi i szeroki zakres usług to tylko niektóre z naszych zalet. Do kluczowych cech, które wyróżniają nas na tle innych firm zaliczamy:
- Tworzenie optymalnych i nietypowych rozwiązań dla klienta.
- Holistycznie i interdyscyplinarnie podejście do realizowanych projektów.
- Dużą elastyczność i zdolność do szybkiego reagowania na zmieniające się wymagania.
- Rozbudowany, wysoko wykwalifikowany zespół ekspertów z różnych dziedzin otwarty na współpracę.
Proponowane przez nas rozwiązania są szyte na miarę Klienta. Strategię działań zawsze dobieramy tak, aby była jak najbardziej zgodna z wizją Klientów, a jednocześnie dbamy o spełnienie wymagań regulacyjnych.
Uważamy, że wielopłaszczyznowa analiza projektu i wyzwań jakie projekt za sobą pociąga stanowi jeden z kluczy do sukcesu. Dlatego też do realizowanych projektów angażujemy ekspertów z wielu dziedzin i przygotowujemy kompleksowe strategie działań, swego rodzaju mapy drogowe, dzięki którym będąc na samym początku projektu wiemy co czeka nas i Klienta, i na co się przygotować w drodze do celu.
Nasze zespoły cechuje elastyczność nie tylko w kontekście prowadzonych projektów i zmieniających się koncepcji Klienta, ale również w kontekście ciągłego dostosowywania się do zmieniających się wymagań i aktów prawnych.
Cenimy sobie przejrzystą współpracę i jesteśmy otwarci na dialog z Klientem. Wiemy jak ważna w osiągnięciu sukcesu jest komunikacja. Dlatego też w trakcie pracy zawsze bierzemy pod uwagę wizję Klienta i jesteśmy dostępni do kontaktu kiedy tylko zajdzie taka potrzeba.
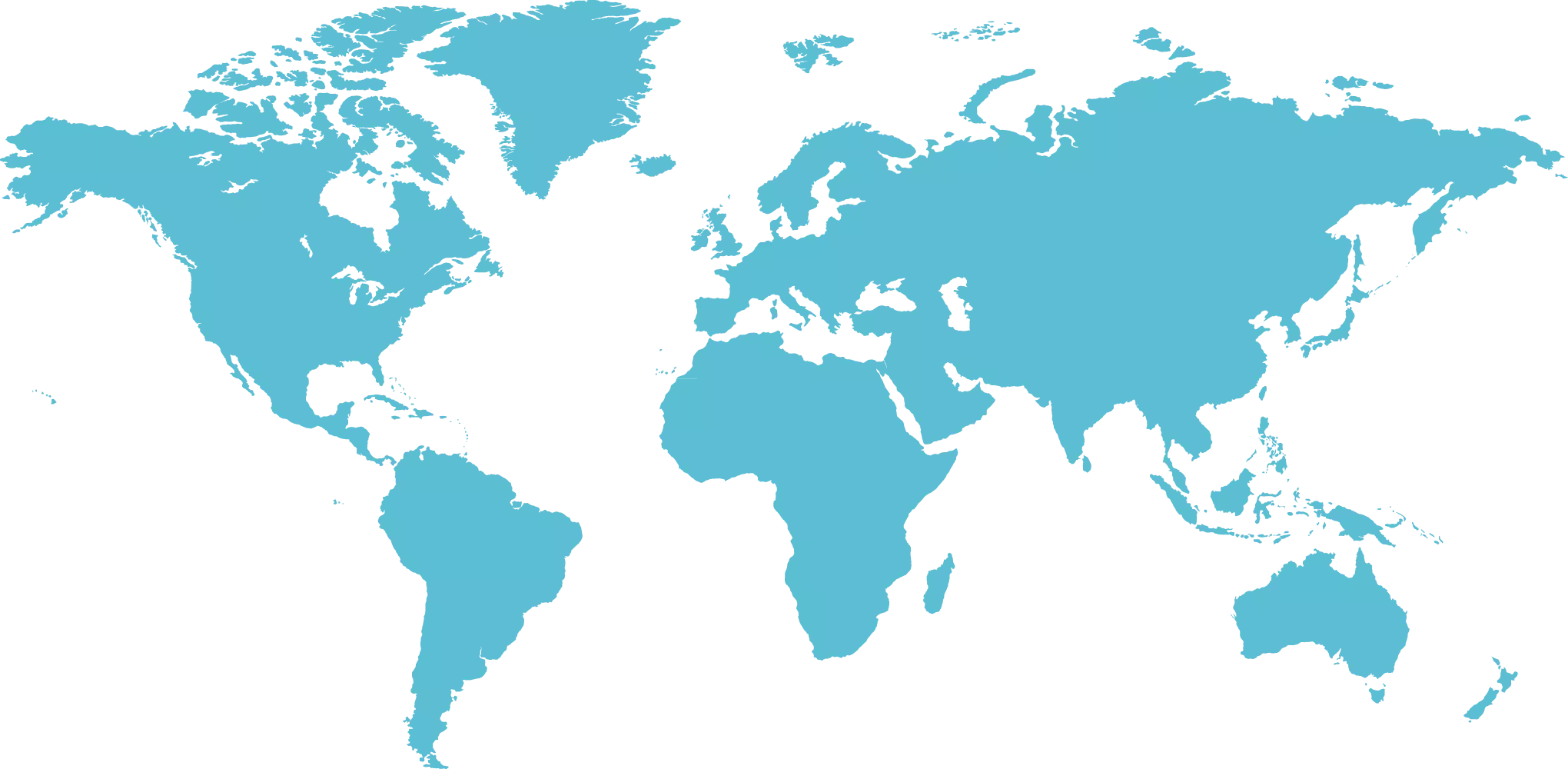
Nasi klienci
Mamy przyjemność realizować projekty farmaceutyczne dla Klientów z całego świata. Wśród nich są firmy zarówno z Polski i innych części Europy, jak i z Azji i Regionu Pacyfiku, Afryki, Ameryki Północnej i Południowej czy też z Kanady. Od lat wspieramy przemysł farmaceutyczny, jak również przedstawicieli świata nauki. Współpracujemy z firmami generycznymi jak i innowacyjnymi, w tym biotechnologicznymi. Nasze usługi doradcze kierujemy do dużych koncernów farmaceutycznych oraz małych i średnich przedsiębiorstw. Z chęcią wspieramy Start-upy oraz małe spółki innowacyjne. Na swoim koncie mamy wspólne projekty z wytwórcami szczepionek i produktów krwiopodobnych, a także z wytwórcami API. Naszymi Klientami są też producenci wyrobów medycznych, produktów weterynaryjnych, kosmetyków i suplementów diety. Do grona naszych Klientów zaliczają się również Uczelnie Wyższe, Instytuty Badawcze i inne jednostki naukowe.
Przez te wszystkie lata zrealizowaliśmy z sukcesem wiele różnych projektów farmaceutycznych dla różnej wielkości firm i organizacji. Dzięki temu zdobyliśmy cenne doświadczenie i poszerzyliśmy horyzonty, ucząc się przy tym, że warto podejmować ryzyko i szukać nieoczywistych rozwiązań. Serdecznie zapraszamy do kontaktu z naszymi ekspertami.
- Europa
- Afryka
- Ameryka Północna
- Azja i region Pacyfiku
- Ameryka Południowa
- Kanada